1.Abstract
Health and Safety policies have led to the recommendation of alternative tests to evaluate the resistance to dichloromethane to estimate the degree of gelation of rigid PVC pipes (PVC-U).
In this work, we confirm that DSC is the best alternative method for evaluating the degree of gelation of rigid PVC pipes, using the relation between the enthalpy of fusion of primary and secondary crystallites, which were measured from respective endotherms.
The suitability of this method is established by comparison with experimental results obtained with the test for determining the resistance to dichloromethane. Indeed, this method has proven to be fairly reliable and is the only one that allows the quantification of the degree of gelation.
Keywords: PVC-U; Degree of gelation; Dichloromethane; Differential scanning calorimetry; Tensile properties.
1.Introduction
For several years, both the manufacturers of unplasticized poly(vinyl chloride) pipe systems and the test laboratories have been facing problems with regard to the performance of gelation tests on PVC-U pipes, due to the Safety & Health policies related to use of dichloromethane (DCM); a solvent that has been traditionally employed for this purpose [1]. Indeed, this substance is included in the Community Rolling Action Plan [2] and some uses of this substance have been restricted under REACH [3].
Moreover, this test may present some technical issues as regards DCM quality, chamfer preparation and the machining effect on pipes during the angular roughing. These issues are due to the aspects as follows: the likely heat effect (as a result of friction), the inaccuracy in the quantification of the dichloromethane attack, as well as to the frequent irregular geometry of the attacked zone(s) across the chamfer width. Lastly, it seems that the chemical attack of DCM is only observed on pipes with a degree of gelation just below about 50%, which is confirmed in the present work.
The latest versions of EN and EN ISO standards for PVC-U pipe systems [4-7], contain, besides the dichloromethane resistance test (of which the results prevail in case of dispute), two alternative tests for determining the degree of gelation. Hence, the two tests as follows can be adopted by the producer for carrying out factory production control, in compliance with the national regulations or the internal health and safety policies: the uniaxial tensile test [8, 9] and the measurement of onset temperature (also known as characteristic temperature) by differential scanning calorimetry (DSC) [10].
Actually, the alternative test preferred is the tensile test, either due to it being more expedient or due to the test equipment being most commonly available in the laboratories carrying out the quality control of plastic pipes. Nonetheless, despite the manufacturers’ preference, both the specification standards for pipes [4-7] and the test standard [8, 9], contain some requirements that are not universally satisfied by pipes for non-pressure applications (as will be addressed later in this article)
Moreover, the present work also shows that the method of preparation of tensile test specimens may have a negative influence on the tensile elongation at break. This method may introduce defects during machining, which are not intrinsic to the material, and which may lead to premature failure.
These features contribute negatively to the adoption of the tensile method as a preferential alternative method.
The second alternative method proposed by ISO pipe standards is based on the specification for ‘Onset temperature’, which establishes the limits Tonset ≥ 185°C for PVC-U pressure pipes (EN ISO 1452-2) and Tonset ≥ 180°C for sewage PVC-U pipes (EN ISO 1401-1, last revision).
The request for a value of onset temperature by DSC not less than 185 °C for PVC-U pressure pipes also limits the adoption of ISO 18372-1 [10] as a valid method. Indeed, this value may be higher for the new lead free formulations, which require a lower mass temperature to achieve a good gelation level [11], and thus it may not be adequate for all PVC formulations.
However, it is known that the degree of gelation of rigid PVC pipes can be assessed by the relation between the enthalpy of fusion of primary and secondary crystallites, which are measured from respective endotherms by DSC, when the general principles established in ISO 18372-2 [12] are adopted.
In this work, the degree of gelation of rigid PVC pipes was evaluated using these different methods. This has made it possible to confirm that the method based on the relation between the enthalpy of fusion of primary and secondary crystallites is the best alternative method to DCM for this assessment.
1 Experimental
1.1 Materials
9 different PVC samples were prepared from rigid PVC pipes, both for water supply and for buried and above-ground drainage and sewerage, with and without pressure [4-7]. All these samples were characterized by dichloromethane resistance tests and tensile properties. However, only 5 of these samples were characterized by DSC.
Some pipes, with a diameter less than 110 cm, which were used for tensile tests, were softly heated above glass transition temperature, near 95ºC, and then subjected to pressure, so as to be flattened for specimen preparation.
The samples for DSC testing were extracted from the walls of the pipes by a hollow punch, and afterwards were cut into layers (core, inner and outer surfaces) and cleaned prior to testing.
All samples and test specimens were kept for at least 24h at a temperature of (23 ± 2) ºC and a relative humidity of (50 ± 5) %.
1.2 Performance under dichloromethane action
The dichloromethane resistance test is a qualitative evaluation performed in compliance with standard ISO 9852 [1]. The specimens were previously chamfered and immersed in a specific stainless-steel container equipped with a cooling system and an exhaust hood.
The collected samples (both DCM attacked and not attacked) were stored and, subsequently, used for comparison with the results obtained from other alternative methods (traction and DSC).
1.3 Tensile proprieties
Tensile specimens of type 1 of ISO 6952-2 [9] were prepared from 7 different samples, using 3 different machining conditions:
- A: manual machining with spindle, dye and milling cutter, without cooling;
- B: computer numerical control (CNC), rotary milling machining;
- C: automatic machining with spindle, dye and milling cutter, with
The tensile tests were carried out using a calibrated Universal Mechanical Testing Machine Instron, Model 4467, with a Class 0.5 30 kN load cell capacity. The tests were performed using a test speed of 5 ± 1 mm/min in a conditioned room at a temperature of (23 ± 2) ºC and a relative humidity of (50 ± 5) %.
1.4 DSC characterization
DSC specimens were prepared from 5 different samples, which were selected from the ones used in the tensile and dichloromethane resistance tests.
The DSC specimens tested were previously weighed in a calibrated balance METTLER AE240 (R = ± 0,01 mg) and the DSC measurements were carried out using a temperature and enthalpy calibrated DSC 2920 MTDSC from TA Instruments. The calibration procedure was as described elsewhere [13].
The scans were carried out in the range from 20ºC to 260ºC, at a heating rate of 25 ± 1
ºC/min1 and using 20 ± 5 ml/min of N2 as purging gas.
DSC tests were performed on two specimens from each sample, which were extracted from random points of the outer surface of the samples. A temperature of 230ºC was selected as the upper limit for the integration of endotherm B.
4 Results and discussion
4.1 Dichloromethane tests
The DCM attack of PVC-U pipes tested by this conventional method may occur on the whole zone of the chamfer that has been immersed in the dichloromethane or only in certain disperse spots. In certain situations, when the degree of gelation is very low, a generalized attack and consequent dissolution of PVC occurs, which leads to the formation of a paste (Figure 1).
Figure 1
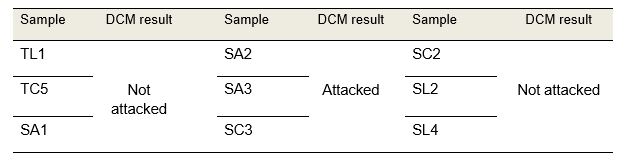
The qualitative results of PVC-U resistance to dichloromethane are presented in Table 1.
Table 1: Results of dichloromethane tests
4.1.1 Tensile tests
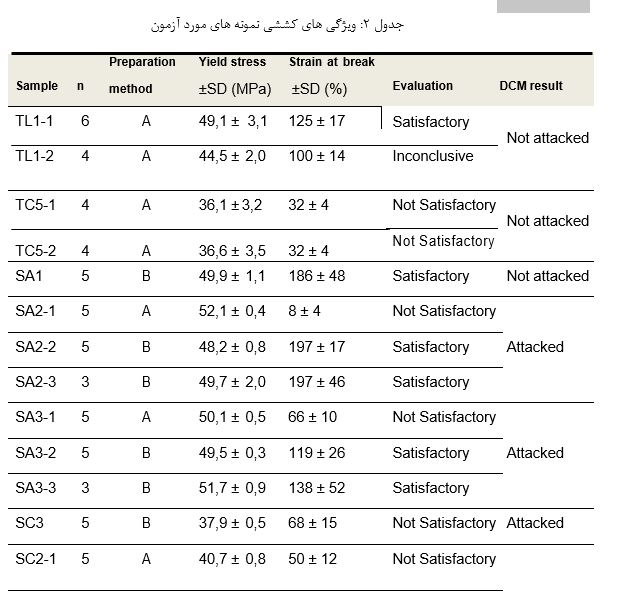
Table 2 presents the results obtained in the tensile tests, including a reference to the method of preparation of test specimens, as well as the respective evaluation, by taking into account both the elongation at break (≥ 80%) and the maximum stress (≥ 45MPa) requirements established in the test standard [8-9] and in the specifications for PVC-U pipes, fittings and system [4-7]. Table 2 also includes a reference to DCM results for comparison purposes.
As can be seen in this table, some tensile results are inconclusive because, although the yield stress are below the threshold value of acceptance criteria established in the standards, they are very close and can be considered satisfactory by rounding since the expanded uncertainty of the test method is in the range of 0.4 MPa. There is also evidence of contradictory results between tensile and dichloromethane resistance acceptance criteria on some samples. Moreover, in some samples, inconsistency in the strain at break between specimens belonging to the same sample is also evident, which shows the influence of the machining conditions of the tensile test specimens on the elongation at break.
Table 2: Tensile proprieties of samples tested
4.1 DSC tests
Standard ISO 18373 [10], [12] includes figures illustrating typical DSC thermograms, which show two endothermic peaks in DSC patterns of processed rigid PVC; a feature that was identified for the first time by Gilbert and Vyvoda.[14].
Part 1 of the ISO standard, that is at the basis of the DSC method proposed for evaluating the degree of gelation of PVC as an alternative to the DCM method, focuses on the onset temperature (or PVC “processing temperature”) located between the first endotherm (“A”), attributed to the melting of secondary crystallites formed as a result of processing, and the second endotherm (“B”), attributed to primary crystallites.
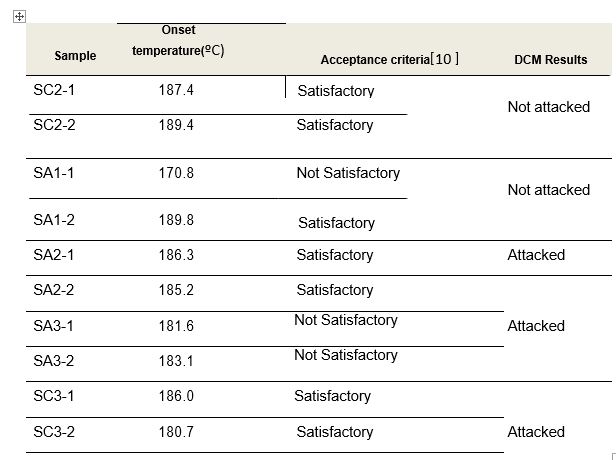
Table 3 presents the results of the onset temperature obtained in the DSC method as well as the DCM results for comparison purposes.
As table 3 shows, the ‘Onset’ temperatures do not match the DCM results in all cases. Actually, they give contradictory results for the two specimens tested from one sample (SA2), and lead to opposite acceptance criteria on both specimens tested from two other samples (SA1 and SC3).
Table 3: Comparison of results of the ‘onset’ temperature determined by DSC and DCM tests
Part 2 of the same ISO standard allows for a measurement of the enthalpy for the smaller of the two endotherms that are observed on a typical PVC DSC plot (“A”), but it does not establish a measurement procedure for obtaining the enthalpy for the second endotherm (“B”).
Although some researchers [15, 16] propose a percent gelation calculation, which is exclusively based on endotherm A, by comparing it with endotherm A for a 100 percent gelled sample (G = ∆KA x100)2, and hence avoiding the uncertainty of the endpoint ∆KAnas determination of endotherm B, this method has not yet been well established.
Nevertheless, several studies [15-20] have been published which describe a different procedure for calculating the “percent gelation” of the PVC pipe material. This procedure consists of measurements of the enthalpy of both the lower and upper endotherms (“A”and “B”) of the DSC thermogram (G = ∆KA x100) and, therefore, it only requires one
∆KA+∆KB
scanning of PVC pipes, because the second endotherm is already present in processed PVC pipes.
Figure 2 shows a typical curve obtained by DSC, from which it is possible to calculate the degree of gelation based on the enthalpy ratio of the lower and upper temperature endotherms (“A” and “B”).Table 4 presents the degree of gelation calculated with this method on all samples tested.
Figure 2
Table 4: Comparison of the degree of gelation calculated by the enthalpy ratio and results of the DCM test
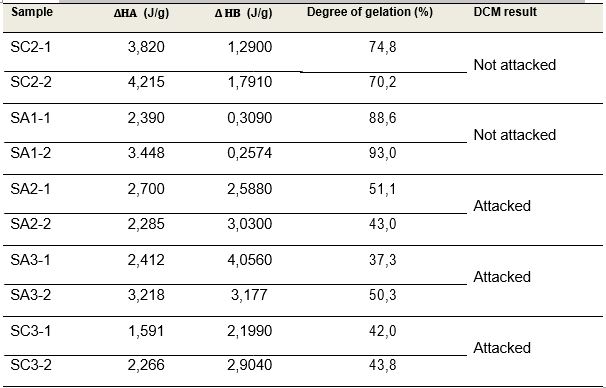
As Table 4 shows, the degree of gelation calculated by the enthalpies of both the lower and upper endotherms (“A” and “B”), are in agreement with the DCM results, hence confirming that the PVC withstands a dichloromethane attack below about 50% of the degree of gelation.
4 Conclusions
It has been shown that the tensile method is not good enough for evaluating the degree of gelation and cannot be considered as a preferential alternative method for replacing the dichloromethane resistance test, due to the following reasons:
- it does not always lead to results consistent with those obtained by the DCM method;
- it can give contradictory results on different specimens of the same sample, which shows that the method of preparation of tensile test specimens may have a negative influence on the tensile elongation at break, probably due to the introduction of defects by machining, which are not visible to the naked eye;
- certain “good” PVC-U pipes are not able to present a yield stress in compliance with the requirements established in the European standard EN ISO 3259-2 [9].
The specification standards [4-6] establish a different PVC content limit, and a reduction in the maximum stress in the tensile test below 45 MPa is expected to occur, as the PVC content is reduced and the CaCO3 content is increased. This has been demonstrated by a study performed by Portuguese manufacturers, in which it was confirmed that the PVC-U pipes show an elongation at break above 110%, but presented reduced values of stress at break, which are in compliance with the PVC content limit 3 . Although it is generally accepted that calcium carbonate has a negative influence on the tensile strength, it is also known that this filler has little or negligible influence on both the gelation and the performance of PVC-U pipes for non-pressure applications.
Thus, the specification standards for pipes [4-7], as well the test standard ISO 6259 [8, 9] contain some requirements that are not universally satisfied by pipes for non-pressure applications and, consequently, it is not possible to meet the mentioned requirements. Therefore, the complementary test is no longer appropriate for such cases.
It is also concluded that the same tensile specimens, prepared by methods A and B, produce opposite results. This leads to the conclusion that method A may use deficient machining conditions, probably due to the lack of cooling fluid, which produces excessive spot heating during machining of test specimens. This feature may lead to unsatisfactory results with erroneous evaluation of the material and final product . Therefore, when the test results are unsatisfactory, further evaluation must be done so as to confirm whether the poor results are due to the preparation method.
On the other hand, the alternative methods to DCM, based on the determination of ‘Onset’ temperatures by DSC, are also inadequate because the results do not always match the DCM results. Nonetheless, the degree of gelation based on the enthalpy ratio of the lower and upper temperature endotherms (“A” and “B) is in agreement with the DCM results, when the PVC shows a dichloromethane attack about 50% below the degree of gelation.
At the outset, the DSC method could be considered to be less representative than the tensile method, because it only gives the possibility of characterising small amounts of samples collected on several parts of the pipe, which could lead to inconsistent conclusions between the two methods under study, if different zones of the pipe were to be analyzed. Nonetheless, despite this limitation, we were able to confirm that DSC is the method with the greatest potential to estimate the percent gelation of the processed PVC materials, due to its sensitivity and ability to describe crystallite melting in a quantitative way (instead of only qualitative), hence displaying good agreement with the DCM test. Additionally, since the thermal protocol is in a ramp (non-isothermal), the time to complete a test is relatively short and it makes possible evaluation of either specific or localized zones of the PVC-U pipe. The non-use of aggressive chemical agents is also an advantage in terms of health and safety . Therefore, the inconsistent conclusions are mainly a consequence of the heterogeneous micro structure of the PVC-U pipe.
However, to consolidate these conclusions and to ensure the preferential adoption of the DSC method for gelation assessment of U-PVC pipes, some improvements must be performed to increase the reproducibility and repeatability of the DSC method [16], particularly as regards some aspects that could be a significant source of errors. These aspects refer to sampling procedures, sample inhomogeneity (due to poor mixing of additives and/or poor processing, causing interference with the DSC curve due to spurious peaks), the possible degradation of PVC during DSC testing above 210ºC, and uncertainty in the determination of the endotherm endpoint.
Acknowledgments
The authors would like to thank the pipe manufacturers FERSIL and POLITEJO for their valuable support. The authors would also like to acknowledge the financial support of LNEC in the scope of P2I research project “Behavior and performance of plastic products, polymer materials with recycled and bio-composites with application in construction (ECOPOL)”.

Figure 2: DSC thermogram for specimen 2 of sample SC2

Evaluating the gelation degree of PVC-U pipes. Comparison between currently available methods
HIGHLIGHTS
The paper shows that both two alternative tests proposed in the last versions of EN and EN ISO standards for evaluating gelation degree of PVC-U pipe systems are not the most appropriate to replace the DCM test, due to several issues.
The paper confirms that the chemical attack of DCM (which is the conventional adopted method in the standards) is only observed on pipes with a gelation degree just below about 50%,
The paper confirms that DSC is the best alternative method for evaluating the gelation degree of rigid PVC pipes, using the relation between the enthalpy of fusion of primary and secondary crystallites, which were measured from respective endotherms.










